The Movement of Nirogen From the Environment to Living Things and Back Again Is Called the
half-dozen Biogeochemical Cycles
After studying this affiliate, you lot should be able to:
- Explain the concept of net master productivity
- Explain the reasons why different biomes accept different levels of primary productivity
- Discuss the biogeochemical cycles of water, carbon, nitrogen, and phosphorus
- Explain how human being activities have impacted these cycles and the resulting potential consequences for Earth
Autotrophs, such equally plants, algae, and photosynthetic bacteria, are the free energy source for a majority of the world's ecosystems. These ecosystems are ofttimes described by grazing and detrital food webs. Photoautotrophs harness the Sun's solar energy by converting it to chemical free energy in the form of ATP (and NADP). The free energy stored in ATP is used to synthesize complex organic molecules, such as glucose. The rate at which photosynthetic producers incorporate free energy from the Sun is called gross master productivity . However, not all of the energy incorporated by producers is bachelor to the other organisms in the food spider web because producers must also grow and reproduce, which consumes energy. Internet chief productivity (NPP) is the energy that remains in the producers after accounting for these organisms' respiration and heat loss. The cyberspace master productivity is and then available to the primary consumers at the next trophic level. Net main productivity is often measured by calculating the biomass of the organisms in an area, which is the dry weight of all the organic matter in an organism. Dry weight is used considering the water stored in the cells of an organism can vary profoundly depending on ecology weather condition.
Net principal productivity varies profoundly across biomes, as seen in Figure ane. Estuaries, or regions where rivers meet the ocean, accept the highest NPP on World. This is largely because rivers bear a constant supply of nutrients from upstream and deposit them into the sea, providing support for a large multifariousness of plants, animals, and other organisms. On land, the nigh productive biomes are swamps and tropical rain forests. This is because these areas take high amounts of available wet and high temperatures. Plants in warm, moist biomes accept a longer growing flavour and rarely lack h2o. Moderate amounts of NPP are found in grasslands and temperate forests, or forests with an intermediate temperature and precipitation, such as the hardwood forests of N America. Terrestrial biomes with the lowest NPP are deserts and tundra. Tundras are treeless chill biomes in which the subsoil is permanently frozen as permafrost. Lack of h2o in deserts and cold temperatures in tundras severely limit the ability of plants to thrive.

Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving every bit heat during the transfers betwixt trophic levels. Rather than flowing through an ecosystem, the matter that makes upward living organisms is conserved and recycled. The 5 most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, and phosphorus—take a variety of chemical forms and may exist for long periods in the temper, on land, in water, or below World's surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a office in the cycling of elements on Earth. Because geology and chemical science take major roles in the study of this process, the recycling of inorganic thing between living organisms and their nonliving surroundings is called a biogeochemical cycle .
The chemicals moving through a biogeochemical cycle may be stored in i identify for a few hours, a few days, a few years, or even millions of years. The places where these chemicals are stored are known as reservoirs, and the amount of fourth dimension spent in each reservoir is known every bit residence time. The processes that move chemicals from i reservoir to another are known equally fluxes or flux mechanisms. For example, water may exist stored in the ocean reservoir and motion to the atmosphere reservoir through the flux mechanism of evaporation.
Water, which contains hydrogen and oxygen, is essential to all living processes. The hydrosphere is the area of Earth where water motility and storage occurs: every bit liquid water on the surface (rivers, lakes, oceans) and beneath the surface (groundwater) or water ice, (polar water ice caps and glaciers), and equally h2o vapor in the temper. Carbon is found in all organic macromolecules and is an of import constituent of fossil fuels. Nitrogen is a major component of our nucleic acids and proteins and is disquisitional to man agriculture. Phosphorus, a major component of nucleic acids, is one of the primary ingredients (forth with nitrogen) in artificial fertilizers used in agriculture, which has environmental impacts on our surface water.
The cycling of these elements is interconnected. For example, the movement of water is critical for the leaching of nitrogen and phosphate into rivers, lakes, and oceans. The ocean is also a major reservoir for carbon. Thus, mineral nutrients are cycled, either apace or slowly, through the entire biosphere betwixt the biotic and abiotic world and from one living organism to another.
The Water Bike
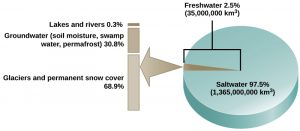
H2o is essential for all living processes. The human torso is more than 1-half h2o and human cells are more than seventy pct h2o. Thus, well-nigh land animals need a supply of fresh water to survive. Of the stores of water on World, 97.5 percent is table salt water (Figure 2). Of the remaining h2o, 99 percent is locked as underground water or ice. Thus, less than one percent of fresh water is nowadays in lakes and rivers. Many living things are dependent on this pocket-size amount of surface fresh water supply, a lack of which tin take important effects on ecosystem dynamics. Humans, of class, have developed technologies to increase water availability, such every bit earthworks wells to harvest groundwater, storing rainwater, and using desalination to obtain potable water from the bounding main. Although this pursuit of beverage water has been ongoing throughout man history, the supply of fresh h2o continues to be a major issue in modern times.
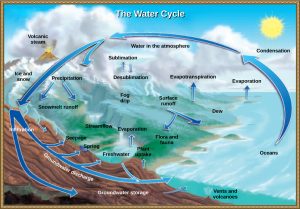
The various processes that occur during the cycling of h2o are illustrated in Figure 3. The processes include the following:
- evaporation and sublimation
- condensation and precipitation
- subsurface water menses
- surface runoff and snowmelt
- streamflow
The water cycle (too known as the hydrologic bike) is driven by the Sun's free energy every bit it warms the oceans and other surface waters. This leads to evaporation (water to water vapor) of liquid surface water and sublimation (ice to water vapor) of frozen water, thus moving large amounts of water into the temper every bit water vapor. Over time, this water vapor condenses into clouds every bit liquid or frozen droplets and eventually leads to precipitation (rain or snowfall), which returns h2o to Earth's surface. Pelting reaching Globe's surface may evaporate once again, period over the surface, or percolate into the basis. Most easily observed is surface runoff: the period of fresh water either from rain or melting ice. Runoff can make its mode through streams and lakes to the oceans or catamenia directly to the oceans themselves.
In about natural terrestrial environments rain encounters vegetation before it reaches the soil surface. A significant percentage of water evaporates immediately from the surfaces of plants. What is left reaches the soil and begins to movement down. Surface runoff will occur just if the soil becomes saturated with water in a heavy rainfall. Most h2o in the soil will be taken up by institute roots. The plant will utilize some of this water for its own metabolism, and some of that will find its way into animals that eat the plants, but much of it volition be lost back to the atmosphere through a procedure known as evapotranspiration. H2o enters the vascular organisation of the found through the roots and evaporates, or transpires, through the stomata of the leaves. Water in the soil that is non taken up by a establish and that does not evaporate is able to percolate into the subsoil and bedrock. Here it forms groundwater.
Groundwater is a significant reservoir of fresh water. Information technology exists in the pores between particles in sand and gravel, or in the fissures in rocks. Shallow groundwater flows slowly through these pores and fissures and somewhen finds its way to a stream or lake where it becomes a part of the surface water again. Streams exercise not flow because they are replenished from rainwater directly; they flow considering there is a abiding inflow from groundwater beneath. Some groundwater is found very deep in the bedrock and can persist there for millennia. Virtually groundwater reservoirs, or aquifers, are the source of drinking or irrigation water drawn up through wells. In many cases these aquifers are beingness depleted faster than they are being replenished by h2o percolating downwardly from above.
Rain and surface runoff are major ways in which minerals, including carbon, nitrogen, and phosphorus are cycled from land to water. The environmental effects of runoff volition be discussed later every bit these cycles are described.
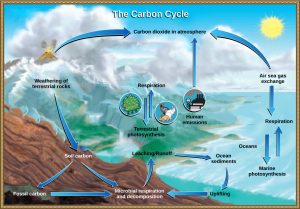
The Carbon Cycle
Carbon is the fourth almost arable element in living organisms. Carbon is present in all organic molecules, and its part in the structure of macromolecules is of primary importance to living organisms. Carbon compounds contain energy, and many of these compounds from plants and algae have remained stored as fossilized carbon, which humans use as fuel. Since the 1800s, the use of fossil fuels has accelerated. As global demand for Earth's limited fossil fuel supplies has risen since the offset of the Industrial Revolution, the corporeality of carbon dioxide in our atmosphere has increased as the fuels are burned. Carbon dioxide is known equally a greenhouse gas because it is a major correspondent to global warming.
The carbon cycle is most easily studied as two interconnected subcycles: ane dealing with rapid carbon exchange amidst living organisms and the other dealing with the long-term cycling of carbon through geologic processes. The entire carbon wheel is shown in Figure iv.
The Biological Carbon Cycle
Living organisms are continued in many means, even betwixt ecosystems. A adept example of this connection is the exchange of carbon between heterotrophs and autotrophs within and betwixt ecosystems by way of atmospheric carbon dioxide. Carbon dioxide is the basic building block that autotrophs use to build multi-carbon, high-energy carbohydrate compounds during the process of photosynthesis. The free energy harnessed from the Sun is used past these organisms to class the covalent bonds that link carbon atoms together. These chemical bonds store this energy for afterwards use in the procedure of cellular respiration. Almost terrestrial autotrophs obtain their carbon dioxide directly from the temper, while marine autotrophs acquire information technology in the dissolved class (carbonic acid, HCOthree –). Yet the carbon dioxide is caused, a byproduct of fixing carbon in organic compounds is oxygen. Photosynthetic organisms are responsible for maintaining approximately 21 percent of the oxygen content of the atmosphere that nosotros observe today.
The partners in biological carbon exchange are the heterotrophs (especially the primary consumers, largely herbivores). Heterotrophs acquire the high-energy carbon compounds from the autotrophs by consuming them and breaking them downwards by cellular respiration to obtain cellular energy, such as ATP. The nigh efficient type of respiration, aerobic respiration, requires oxygen obtained from the atmosphere or dissolved in water. Thus, in that location is a constant exchange of oxygen and carbon dioxide between the autotrophs (which need the carbon) and the heterotrophs (which need the oxygen). Autotrophs as well respire and swallow the organic molecules they form: using oxygen and releasing carbon dioxide. They release more oxygen gas as a waste matter production of photosynthesis than they utilise for their own respiration; therefore, there is excess bachelor for the respiration of other aerobic organisms. Gas exchange through the atmosphere and h2o is 1 way that the carbon cycle connects all living organisms on Earth.
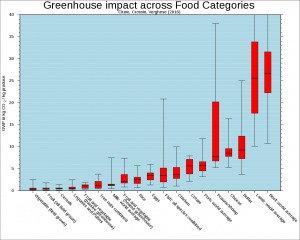
Carbon is also added to the atmosphere by the agriculture practices of humans. The large number of state animals raised to feed Earth's growing human population results in increased carbon dioxide levels in the atmosphere, largely because more land is required to raise livestock animals than to raise plants (Figure 5). This leads to increased deforestation as trees are cut downwardly to brand room for farms. Cattle take an even greater impact on climate alter than other animals raised for nutrient because their unique digestive system also produces methane (CHiv), a greenhouse gas that is at least 20 times stronger in its warming potential than carbon dioxide. Although much of the fence about the future effects of increasing atmospheric carbon on climatic change focuses on fossils fuels, scientists are growing more than concerned almost the impacts of raising an ever growing number of animals for food. Scientists must likewise take natural processes, such as volcanoes, constitute growth, soil carbon levels, and respiration, into account equally they model and predict the time to come bear upon of homo changes to the carbon cycle.
The Biogeochemical Carbon Bicycle
The movement of carbon through land, water, and air is complex, and, in many cases, information technology occurs much more than slowly geologically than the motility between living organisms. Carbon is stored for long periods in what are known as carbon reservoirs, which include the atmosphere, bodies of liquid h2o (generally oceans), ocean sediment, soil, rocks (including fossil fuels), and Earth'due south interior.
As stated, the atmosphere is a major reservoir of carbon in the form of carbon dioxide that is essential to the process of photosynthesis. The level of carbon dioxide in the atmosphere is greatly influenced by the reservoir of carbon in the oceans. The exchange of carbon betwixt the temper and water reservoirs influences how much carbon is found in each, and each one affects the other reciprocally. Carbon dioxide (CO2) from the atmosphere dissolves in water and, different oxygen and nitrogen gas, reacts with water molecules to form ionic compounds. Some of these ions combine with calcium ions in the seawater to form calcium carbonate (CaCO3), a major component of the shells of marine organisms. These organisms somewhen course sediments on the ocean floor. Over geologic time, the calcium carbonate forms limestone, which comprises the largest carbon reservoir on Earth.
On land, carbon is stored in soil as organic carbon every bit a result of the decomposition of living organisms or from weathering of terrestrial rock and minerals. Deeper nether the ground, at land and at sea, are fossil fuels, the anaerobically decomposed remains of plants that take millions of years to form. Fossil fuels are considered a non-renewable resources because their use far exceeds their rate of germination. A non-renewable resource is either regenerated very slowly or not at all. Some other way for carbon to enter the atmosphere is from land (including land beneath the surface of the ocean) by the eruption of volcanoes and other geothermal systems. Carbon sediments from the ocean flooring are taken deep within Earth by the process of subduction : the movement of one tectonic plate below another. Carbon is released every bit carbon dioxide when a volcano erupts or from volcanic hydrothermal vents.
The Nitrogen Wheel
Getting nitrogen into the living world is difficult. Plants and phytoplankton are non equipped to comprise nitrogen from the atmosphere (which exists as tightly bonded, triple covalent Ntwo) even though this molecule comprises approximately 78 per centum of the atmosphere. Nitrogen enters the living world via complimentary-living and symbiotic bacteria, which contain nitrogen into their cells through nitrogen fixation (conversion of Nii). Cyanobacteria live in most aquatic ecosystems where sunlight is present; they play a fundamental role in nitrogen fixation. Cyanobacteria are able to use inorganic sources of nitrogen to "prepare" nitrogen. Rhizobium bacteria live symbiotically in the root nodules of legumes (such as peas, beans, and peanuts) and provide them with the organic nitrogen they need. Free-living bacteria, such as Azotobacter, are likewise important nitrogen fixers.
Organic nitrogen is peculiarly important to the written report of ecosystem dynamics since many ecosystem processes, such equally master production and decomposition, are limited by the available supply of nitrogen. Equally shown in Effigy 6, the nitrogen that enters living systems past nitrogen fixation is eventually converted from organic nitrogen dorsum into nitrogen gas by bacteria. This process occurs in three steps in terrestrial systems: ammonification, nitrification, and denitrification. First, the ammonification process converts nitrogenous waste product from living animals or from the remains of dead animals into ammonium (NH4 + ) by certain leaner and fungi. Second, this ammonium is then converted to nitrites (NO2 −) by nitrifying leaner, such as Nitrosomonas, through nitrification. Subsequently, nitrites are converted to nitrates (NO3 −) past similar organisms. Lastly, the process of denitrification occurs, whereby bacteria, such equally Pseudomonas and Clostridium, catechumen the nitrates into nitrogen gas, thus allowing it to re-enter the temper.
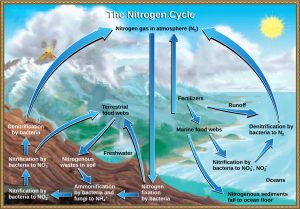
Human activity can release nitrogen into the environment by two primary means: the combustion of fossil fuels, which releases various nitrogen oxides, and by the use of artificial fertilizers (which incorporate nitrogen and phosphorus compounds) in agriculture, which are then washed into lakes, streams, and rivers by surface runoff. Atmospheric nitrogen (other than Ntwo) is associated with several effects on Earth's ecosystems including the production of acid rain (as nitric acid, HNO3) and greenhouse gas effects (as nitrous oxide, North2O), potentially causing climate change. A major effect from fertilizer runoff is saltwater and freshwater eutrophication , a process whereby nutrient runoff causes the overgrowth of algae and a number of consequential issues.
A like process occurs in the marine nitrogen cycle, where the ammonification, nitrification, and denitrification processes are performed by marine bacteria and archaea. Some of this nitrogen falls to the ocean floor equally sediment, which can then be moved to land in geologic time by uplift of World's surface, and thereby incorporated into terrestrial rock. Although the move of nitrogen from rock directly into living systems has been traditionally seen as insignificant compared with nitrogen fixed from the atmosphere, a contempo study showed that this process may indeed be significant and should exist included in any study of the global nitrogen bicycle.
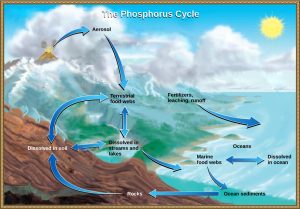
The Phosphorus Bicycle
Phosphorus is an essential nutrient for living processes; it is a major component of nucleic acids and phospholipids, and, as calcium phosphate, makes upward the supportive components of our bones. Phosphorus is oftentimes the limiting nutrient (necessary for growth) in aquatic, particularly freshwater, ecosystems.
Phosphorus occurs in nature every bit the phosphate ion (PO4 3-), plant largely in rocks. Humans extract this phosphate and utilize it in products such as fertilizer and detergents, which can run off into water bodies. In addition to phosphate runoff as a result of human being activity, natural surface runoff occurs when it is leached from phosphate-containing rock by weathering, thus sending phosphates into rivers, lakes, and the sea. This rock has its origins in the body of water. Phosphate-containing ocean sediments class primarily from the bodies of sea organisms and from their excretions. Yet, volcanic ash, aerosols, and mineral dust may also exist meaning phosphate sources. This sediment so is moved to land over geologic time by the uplifting of Earth's surface (Effigy 7).
Phosphorus is also exchanged between phosphate dissolved in the bounding main and marine organisms. The move of phosphate from the ocean to the land and through the soil is extremely slow, with the average phosphate ion having an oceanic residence time between 20,000 and 100,000 years.
Backlog phosphorus and nitrogen that enter these ecosystems from fertilizer runoff and from sewage cause excessive growth of algae. The subsequent death and decay of these organisms depletes dissolved oxygen, which leads to the expiry of aquatic organisms, such as shellfish and finfish. This procedure is responsible for dead zones in lakes and at the mouths of many major rivers and for massive fish kills, which often occur during the summertime months (Figure viii).
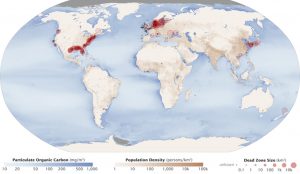
A dead zone is an surface area in lakes and oceans near the mouths of rivers where large areas are periodically depleted of their normal flora and fauna; these zones tin exist caused by eutrophication, oil spills, dumping toxic chemicals, and other human activities. The number of dead zones has increased for several years, and more than 400 of these zones were present as of 2008. One of the worst dead zones is off the declension of the U.s.a. in the Gulf of Mexico: fertilizer runoff from the Mississippi River basin created a dead zone of over 8,463 foursquare miles. Phosphate and nitrate runoff from fertilizers likewise negatively touch on several lake and bay ecosystems including the Chesapeake Bay in the eastern Us.
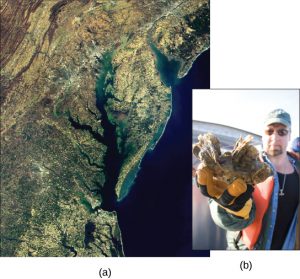
The Chesapeake Bay (Figure 8a) is one of the most scenic areas on Earth; information technology is at present in distress and is recognized as a case study of a declining ecosystem. In the 1970s, the Chesapeake Bay was i of the first aquatic ecosystems to accept identified expressionless zones, which continue to impale many fish and bottom-dwelling species such as clams, oysters, and worms. Several species take declined in the Chesapeake Bay because surface water runoff contains excess nutrients from artificial fertilizer use on country. The source of the fertilizers (with high nitrogen and phosphate content) is not limited to agricultural practices. There are many nearby urban areas and more than 150 rivers and streams empty into the bay that are carrying fertilizer runoff from lawns and gardens. Thus, the pass up of the Chesapeake Bay is a complex issue and requires the cooperation of industry, agriculture, and individual homeowners.
Of particular involvement to conservationists is the oyster population (Effigy 8b); information technology is estimated that more than 200,000 acres of oyster reefs existed in the bay in the 1700s, but that number has now declined to simply 36,000 acres. Oyster harvesting was once a major industry for Chesapeake Bay, but it declined 88%between 1982 and 2007. This refuse was caused not only by fertilizer runoff and dead zones, simply likewise because of overharvesting. Oysters require a certain minimum population density because they must be in close proximity to reproduce. Human activeness has contradistinct the oyster population and locations, thus greatly disrupting the ecosystem.
The restoration of the oyster population in the Chesapeake Bay has been ongoing for several years with mixed success. Not simply practice many people find oysters skillful to eat, only the oysters likewise clean upward the bay. They are filter feeders, and as they swallow, they clean the h2o around them. Filter feeders eat by pumping a continuous stream of water over finely divided appendages (gills in the case of oysters) and capturing prokaryotes, plankton, and fine organic particles in their mucus. In the 1700s, it was estimated that information technology took only a few days for the oyster population to filter the entire volume of the bay. Today, with the changed water conditions, information technology is estimated that the present population would take about a twelvemonth to do the aforementioned job.
The restoration goal is to find a way to increase population density and then the oysters can reproduce more efficiently. Many disease-resistant varieties (developed at the Virginia Found of Marine Science for the College of William and Mary) are now available and have been used in the construction of experimental oyster reefs. Efforts by Virginia and Delaware to clean and restore the bay have been hampered because much of the pollution entering the bay comes from other states, which emphasizes the need for interstate cooperation to gain successful restoration.
The new, hearty oyster strains have too spawned a new and economically viable industry—oyster aquaculture—which not only supplies oysters for food and turn a profit, just also has the added benefit of cleaning the bay.
Attribution
"Biogeochemical Cycles" in OpenStax Concepts of Biological science, modified by Sean Whitcomb. License: CC BY
Source: https://open.maricopa.edu/environmentalscience/chapter/biogeochemical-cycles/
0 Response to "The Movement of Nirogen From the Environment to Living Things and Back Again Is Called the"
Post a Comment